Mrs. Rukmini Zainal Abidin, opened the first nationally indigenous owned pharmacy.
-
1950
-
1954
Mr. Zainal Abidin, opened his first indigenous pharmaceutical factory, PT ABDI.
-
1970
Mrs Rukmini and Mr. Zainal Abidin opened their second factory,
PT TUNGGAL, and began a period of long-standing collaboration with various pharmaceutical multinational companies as their appointed toll manufacturer. -
1975
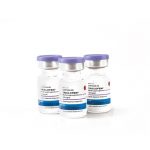
PT TUNGGAL, began a 30 year collaboration with Upjohn Co. in producing DepoProvera on behalf of Pfizer for the Indonesian market, where Tunggal produced over 120 million vials during this period.
-
1992
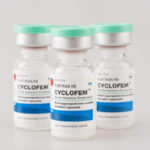
PT TUNGGAL IDAMAN ABDI jointly with Concept Foundation, participated in a global study, and became the licensee manufacturer of the innovator monthly injectable contraceptive, Cyclofem.
-
1994
The merger of PT TUNGGAL and
PT ABDI was established and officially became
PT TUNGGAL IDAMAN ABDI -
2006
PT TUNGGAL IDAMAN ABDI revamped its hormone injectable line, installing its first Bosch compact filling machine.
-
2007
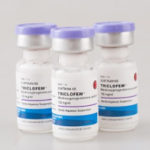
PT TUNGGAL IDAMAN ABDI, launched its own three monthly injectable, Triclofem.
-
2013
PT TUNGGAL IDAMAN ABDI launched its herbal range, Herbatia, consisting of standardised herbal extracts for both post and pre natal treatments.
-
2015
PT TUNGGAL IDAMAN ABDI officially began operating its new dedicated hormone plant, and filed its submission for WHO Pre-Qualification on its three-monthly injectable contraceptives.
-
2016
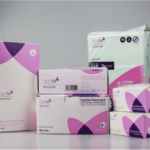
PT TUNGGAL IDAMAN ABDI launched its medical device range, Femitia, offering a range of medical devices aimed for the midwives, such as medical gloves, pregnancy test kit, and syringes.
-
2017
PT TUNGGAL IDAMAN ABDI opened its 45th domestic branch in Jayapura, where we are now present in 31 major cities nationally, serving over 46,000 midwives and all major healthcare outlets throughout Indonesia.
-
2018
PT TUNGGAL IDAMAN ABDI was awarded an Expert Review Panel certification (ERP) by UNFPA which made Triclofem eligible for procurement by WHO related agencies and major NGOs.
-
2020
Celebrating its 50th year anniversary,
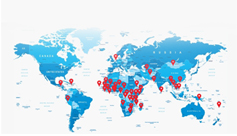
PT TUNGGAL IDAMAN ABDI’s combined export of its monthly and three monthly injectables today stretches to over 50 countries in Asia, Africa and South America. -
2021
PT TUNGGAL IDAMAN ABDI was awarded WHO PQ status for its three monthly injectable contraceptive, Triclofem by WHO.